Acidification: Difference between revisions
BrianNaess (talk | contribs) |
BrianNaess (talk | contribs) |
||
| Line 8: | Line 8: | ||
Recent research suggests that corals will not be able to "acclimate or adapt to maintain sufficient rates of calcification to sustain the reef structure." <ref>Elizabeth D. Crook, Anne L. Cohen, Mario Rebolledo-Vieyra, Laura Hernandez, and Adina Paytan. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. PNAS 2013 : 1301589110v1-201301589.</ref> | Recent research suggests that corals will not be able to "acclimate or adapt to maintain sufficient rates of calcification to sustain the reef structure." <ref>Elizabeth D. Crook, Anne L. Cohen, Mario Rebolledo-Vieyra, Laura Hernandez, and Adina Paytan. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. PNAS 2013 : 1301589110v1-201301589.</ref> | ||
==== Research ==== | |||
"Acidification of the Arctic Ocean is occurring faster than projected, according to new findings published in September 2013 in the journal PLOS ONE (Baseline Monitoring of the Western Arctic Ocean Estimates 20% of Canadian Basin Surface Waters Are Undersaturated with Respect to Aragonite). The increase in rate is being blamed on rapidly melting sea ice, a process that may have important consequences for health of the Arctic ecosystem." <ref>http://soundwaves.usgs.gov/2013/12/research2.html</ref> | |||
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 14:19, 16 January 2014
Ocean Acidification
What is acidification?
Typical ocean pH values range between 7.5 and 8.4. [1] Ocean acidification is a chemical process that, through an increase in the amount of carbon dioxide dissolving in seawater, the pH of the ocean decreases. Carbon dioxide (CO2) and water (H2O) react to form carbonic acid (H2CO3). The formation of acid in this reaction causes an increase in hydrogen ions (H+) which lowers the pH of the ocean and therefore increases acidity. Lower ocean pH caused by acidification can have many harmful impacts on ocean dwelling organisms. Carbonic acid reacts to dissolve carbonate, which is what corals use to make their skeletons. Other organisms that make carbonate structures are oysters, clams, sea urchins, shallow water corals, deep sea corals, and plankton.[2]
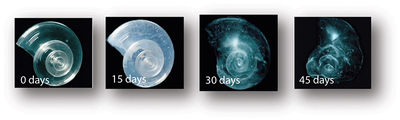
Recent research suggests that corals will not be able to "acclimate or adapt to maintain sufficient rates of calcification to sustain the reef structure." [4]
Research
"Acidification of the Arctic Ocean is occurring faster than projected, according to new findings published in September 2013 in the journal PLOS ONE (Baseline Monitoring of the Western Arctic Ocean Estimates 20% of Canadian Basin Surface Waters Are Undersaturated with Respect to Aragonite). The increase in rate is being blamed on rapidly melting sea ice, a process that may have important consequences for health of the Arctic ecosystem." [5]
References
- ↑ Sumich, James L. An Introduction to the Biology of Marine Life, Seventh Edition. WCB/McGraw Hill. 1999.
- ↑ K.R.N. Anthony, et al. (2008). Ocean acidification causes bleaching loss in coral reef builders. PNAS vol. 105 no. 45.
- ↑ http://www.pmel.noaa.gov/co2/story/What+is+Ocean+Acidification%3F
- ↑ Elizabeth D. Crook, Anne L. Cohen, Mario Rebolledo-Vieyra, Laura Hernandez, and Adina Paytan. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. PNAS 2013 : 1301589110v1-201301589.
- ↑ http://soundwaves.usgs.gov/2013/12/research2.html