Acidification
Ocean Acidification
What is acidification?
Typical ocean pH values range between 7.5 and 8.4. [1] Ocean acidification is a chemical process that, through an increase in the amount of carbon dioxide dissolving in seawater, the pH of the ocean decreases. Carbon dioxide (CO2) and water (H2O) react to form carbonic acid (H2CO3). The formation of acid in this reaction causes an increase in hydrogen ions (H+) which lowers the pH of the ocean and therefore increases acidity. Lower ocean pH caused by acidification can have many harmful impacts on ocean dwelling organisms. Carbonic acid reacts to dissolve carbonate, which is what corals use to make their skeletons. Other organisms that make carbonate structures are oysters, clams, sea urchins, shallow water corals, deep sea corals, and plankton.[2]
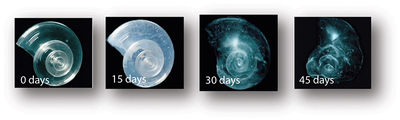
Recent research suggests that corals will not be able to "acclimate or adapt to maintain sufficient rates of calcification to sustain the reef structure." [4]
References
- ↑ Sumich, James L. An Introduction to the Biology of Marine Life, Seventh Edition. WCB/McGraw Hill. 1999.
- ↑ K.R.N. Anthony, et al. (2008). Ocean acidification causes bleaching loss in coral reef builders. PNAS vol. 105 no. 45.
- ↑ http://www.pmel.noaa.gov/co2/story/What+is+Ocean+Acidification%3F
- ↑ Elizabeth D. Crook, Anne L. Cohen, Mario Rebolledo-Vieyra, Laura Hernandez, and Adina Paytan. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. PNAS 2013 : 1301589110v1-201301589.