Macroalgae
Overview
Macroalgae, or seaweed, are a group of diverse organisms in the algae family. They are divided by color into three major phyla; rhodophyta (red), chlorophyta (green), and ochrophyta (brown). Macroalgae is not a plant, as it does not have the reproductive characteristics of plants, and instead is classed as a eukaryotic organism.
The three groups of macroalgae are biologically very distinct. In a study of the medicinal properties of the different phyla of macroalgae, Adarshan, et al noted that the three had very distinct medicinal properties.[1] Rhodophyta have lots of phycobiliproteins, pigments, phycolectins, mycosporine, unsaturated fatty acids, polysaccharides, minerals, and vitamins. Red and green contain lots of bromophenols, phenolic acids and flavonoids. Brown algae contain lots of phlorotannin [1]. All are high in protein, with it making up between 7 and 31% of their dry weight [2]. Macroalgae thus plays an important role in the protein cycle, along with their photosynthetic role, producing oxygen and capturing carbon dioxide.
Macroalgae is benthic (bottom dwelling) attaching either to the seabed, or a hard substrate, like reefs, rocks and mangrove roots. Due to their reliance on photosynthesis, they only grow in the photic zone of water. Common species include caulerpa, codium and ulva (green), hormosira banksii, phyllospor comosa and ecklonia radiata (brown), and crustose corallines, cryptonemia sp and augophyllum delicatum (red).
When the ecosystem is in balance, macroalgae helps sustain coral reef microbiomes. Reef dwelling creatures like fish, crabs and urchin eat macroalgae, while the organism itself helps with nitrogen cycling. Further, crustose calcareous algae are the preferred settlement substrate for a number of coral species, meaning its presence is helpful when there is disruption to existing reefs [3]. However, the ecosystem is not currently in balance. There are a number of reasons for this. For one, macroalgae, as a photosynthetic organism, feeds on carbon dioxide, which is increasingly abundant. Overfishing has resulted in a decline in the populations of herbivorous fish, mammals and invertebrates which naturally feed upon macroalgae. Finally, coastal development has resulted in nutrient loading across the coastline. Studies have found higher concentrations of nitrogen and phosphorus, found in material like fertilizer, have resulted in increased blooms. Of these three factors, coastal development is thought to have the smallest consistent impact [4], however in reef communities with significant runoff, it has perhaps the greatest impact. For instance it was estimated that for about 23% of the Great Barrier Reef, increased water quality would result in a reduction in macroalgal cover by around 39% [5].
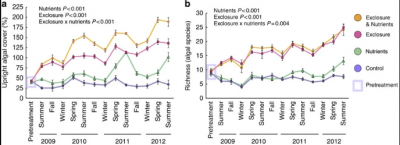

Figure 1. Effect of Herbivore Exclusion on Macroalgae. Graph A measures
macroalgal cover, while b measures species richness[6]
All three of these have resulted in increased macroalgal blooms. Larger blooms result in greater photosynthesis, creating more oxygen for local bacteria. This results in an increase in the population of this oceanic bacteria, which in turn competes with the coral, depriving it of oxygen, hurting it via hypoxia. Further, the macroalgae itself can smother the coral, and transfer algal-associated microbes to the reef [6]. The cumulative result of this was not just a decrease in coral health and population, but a decrease in coral biodiversity [7]. Declining ecosystem biodiversity has the result of making that area more vulnerable to future ecological shocks, meaning that these reef systems have become more at risk from future natural disasters, further climate change, and coral disease. At the same time, macroalgae’s presence, while not totally causal in reef decline, is driven by factors that independently also drive a decline in coral reefs.
This theoretical relationship between macroalgal blooms is not always perfectly clear in the data. In Yawzi Point, St John, scleractinian cover fell from 44.6% (±3.4%) to 5.6% (±1.4%) between 1987 and 2013. At the same time macroalgae cover grew from 2.2% (±0.4%) to 32.9% (±3.7%). At the same time Tektite, St John, saw an increase in macroalgal cover from <12.8% to 28.4% (±1.7%), while scleractinian cover remind reasonably steady, declining from 32.2% (±2.2%) to 28.3% (±1.7%). Importantly, the effect of macroalgae seems smaller when population increases are more limited [8]. Further, there is a large underlying effect of other factors like temperature variation, rainfall and hurricane intensity.
Approaches to Managing Macroalgae
Method 1 - Caribbean King Crabs
Measures need to be taken to prevent the decline of coral reefs that result from macroalgae's increased cover. Various scientists have conducted research studies on approaches to algae management to help combat the increase in algae cover. Jason Spadaro and his team researched one method in the Florida Keys. Spadaro researched the Caribbean king crab: large herbivorous crabs that inhabit the western Atlantic Ocean and the Caribbean. The primary diet for the Caribbean king crab is macroalgae, and they consume macroalgae at rates that are significantly higher than other organisms. However, since their natural density is relatively low, the impact they have on reducing algae cover is close to negligible. As a solution, Spadaro developed a plan to breed about a quarter of a million Caribbean king crabs at his lab each year [9].


Figure 2. Spadaro holding Caribbean King Crab[9]
Spadaro and his team of researchers proceeded to conduct a study on the impact of the Caribbean king crabs on reducing algal cover. The team hypothesized that an increase in crab density would reduce the macroalgae’s dominance, allowing the coral reef community’s balance to be restored. The study involved assigning 12 coral reef locations in the Florida Keys to one of the three experimental groups–four locations in each group. Group 1 served as the control group, group 2 supplied the reefs with crabs, and group 3 involved divers removing seaweed before placing the crabs. The act of removing the seaweed is referred to as scrubbing. Spadaro conducted two of the same tests in order to validate the generality of the results. The team conducted the first test from 2014-2015 and the second test from 2016-2017. The second test took place at a location that was about 13 km away from the first test. Throughout the duration of each test, the researchers monitored the algal cover, coral recovery, and the fish population [10].
Each test yielded the same significant results. For the coral reef locations that only involved the increase in crab density, the crabs contributed to a decline of about 50% of algal cover in comparison to the control group. The locations that included scrubbing before introducing the crabs resulted in a decrease of the algal cover by about 70-80%. The repeatability of the results for the two tests conducted allows the researchers to conclude that the Caribbean king corals have a substantial effect on reducing macroalgae in the coral reef community.
In addition to the three experimental groups, Spadaro included a fourth group when conducting the second test. The team was only responsible for scrubbing; no crabs were supplied to the reef areas. Locations assigned to the experimental group showed inconsistent results for the effectiveness of scrubbing. The absence of macroalgae-eating crabs allowed the final amount of the algal cover to nearly match the initial cover in some areas. In contrast, some areas reported a decrease in algal cover for certain types of algae [10]. Figure 3 summarizes the results from each experimental group in test two.
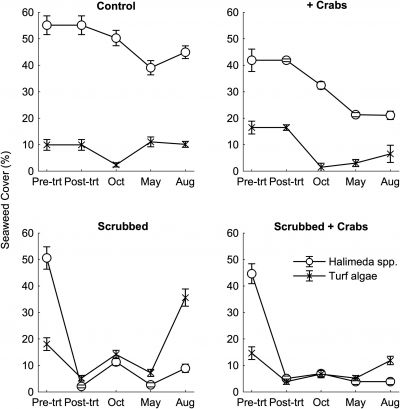

Figure 3. Changes in the Algal Cover for Two Types of Algae on Coral
Reefs during the Second Test[10]
Method 2 – Manual Sea-weeding
While Spadaro and his team report inconsistencies in reducing macroalgae cover through manual seaweed removal, other studies have found positive effects. For example, Hillary Smith and her team of researchers performed a study on manual sea-weeding. The research team established 24 coral reef experimental sites along the coast of Yunbenun, 12 of which were located in Arthur Bay and the remaining 12 in Florence Bay. At each bay, 6 sites were randomly assigned to be control groups, and the other 6 sites had seaweed removed periodically. The study lasted from October of 2018 to October of 2021. Scuba divers placed the collected seaweed into bags in order to weigh the amount of seaweed removed from a field site. Photographic surveys were conducted one day and one week after each removal event. The research team computed statistical analyses based on the diversity index, evenness, and richness of the coral and macroalgal communities [11].
The end of the study revealed promising results for manual sea-weeding. The initial mean macroalgal coverage for the control sites was 87.04%, and the resulting mean coverage remained around the same at 83.39%. For the removal sites, the mean average started at 82.35% and dropped to 19.34%. In addition, the average coral cover increased by about 47% for the removal sites and remained stable for the control groups. The results of the study conclude that periodical manual sea-weeding can have positive impacts on decreasing macroalgal cover and increasing coral recovery. This low-tech, influential method can rapidly improve coral reef communities with the help of local community groups and scientists [11].
Method 3 - Establishment of No-Take Zones
Macroalgae in and of itself is not damaging to a marine environment. It is rather the overgrowth of macroalgae that threatens coral by taking up space and light. A healthy amount of macroalgae can fulfill primary production, food/habitat provision, and reef framework consolidation[11]. Overgrowth of macroalgae is not a natural issue either, but rather a result of a couple of factors: reduced grazing, reduced competition from coral(due to coral death events), and increased nutrient loads[11].
Reduced grazing can be correlated with overfishing. Overfishing leads to less fish, which leads to less grazing and more macroalgae growth that threatens coral populations. Establishment of No-Take Zones offers an ecosystem-based approach to the issues caused by overfishing and overall decline of marine environment health. A marine protected area, referred to as MPA, provides restrictions in order to conserve resources. Establishment of MPAs does not directly affect macroalgae growth, however it can increase fish populations(indirectly leading to less macroalgae). In fact, MPAs that have had the most positive impacts on resources are those that regulate resource extraction, aka fishing [12]. Positive impacts include conservation of exploited species shown through increased density of populations, increased biomass per area, and increased physical size of fish [12]. All of these factors will increase grazing in an area, therefore decreasing macroalgae. Greater populations of bigger fish will have a higher rate of grazing. Additionally, larger female fish will also produce more eggs, so the population growth of no-take-zones compared to unprotected areas is vastly different. The same can be said for male fish, larger male fish are more likely to reproduce and increase the number of fertilized eggs [12]. No-take-zones also foster an environment where survival rates of larvae to juvenile to adult fish are increased.
Reduced competition from coral can also be remedied by establishment of an MPA. Increased biodiversity can result from MPA establishment, as threats to biodiversity are minimized. More biodiversity leads to more competition for macroalgaes. Increased biodiversity has a widespread effect on not only direct competition for the macroalgae, but for the predator/prey relations between species and food web as a whole. This is particularly significant in the populations of parrot fish and urchins who are key grazers [12].
No-Take Zones not only benefit the protected area, but also the surrounding area via the “recruitment effect” and the "spillover effect". In the recruitment effect, larvae are carried by currents outside of the MPA zone and can replenish unprotected populations [12]. In the spillover effect, a similar process can be observed. Juveniles or adults leave the MPA zone due to crowding and also replenish unprotected zone populations [12]. Both of these effects will increase grazing and biodiversity, which then cause decreased macroalgae that threatens coral life.
References
- [↑ 1.0 1.1 Adarshan, Sivakumar, et al.“Understanding Macroalgae: A Comprehensive Exploration of Nutraceutical, Pharmaceutical, and Omics Dimensions.” Plants, vol 13, no. 1, 2024 pp.113. https://doi.org/10.3390/plants13010113]{#cite_note-adarshan-1}
- [↑ Biris-Dorhoi, Elena-Suzana, et al. (2020). "Macroalgae-A Sustainable Source of Chemical Compounds with Biological Activities." Nutrients, 12(10), 3085. https://doi.org/10.3390/nu12103085]{#cite_note-biris-2}
- [↑ El-Manaway, I.M., Rashedy, S.H. (2022). "The Ecology and Physiology of Seaweeds: An Overview." In: Ranga Rao, A., Ravishankar, G.A. (eds) Sustainable Global Resources Of Seaweeds Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-030-91955-9_1]{#cite_note-el-manaway-3}
- [↑ Teichberg, M., Martinetto, P., Fox, S.E. (2012). "Bottom-Up Versus Top-Down Control of Macroalgal Blooms." In: Wiencke, C., Bischof, K. (eds) Seaweed Biology. Ecological Studies, vol 219. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28451-9_21]{#cite_note-teichberg-4}
- [↑ Ridd, P. V., Orpin, A. R., Stieglitz, T. C., & Brunskill, G. J. (2011). "Will reducing agricultural runoff drive recovery of coral biodiversity and macroalgae cover on the Great Barrier Reef?" Ecological Applications, 21(8), 3332–3335. http://www.jstor.org/stable/41417130]{#cite_note-ridd-5}
- [↑ 6.0 6.1 Zaneveld, Jesse R, et al. "Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales." Nat Commun 7, 11833 (2016). https://doi.org/10.1038/ncomms11833]{#cite_note-zaneveld-6}
- [↑ De'ath, G., & Fabricius, K. (2010). "Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef." Ecological Applications, 20(3), 840–850. http://www.jstor.org/stable/27797851]{#cite_note-df-7}
- [↑ Tsounis, Georgious, & Edmunds, Peter J. 2017. "Three decades of coral reef community dynamics in St. John, USVI: a contrast of scleractinians and octocorals." Ecosphere 8(1):e01646. 10.1002/ecs2.1646]{#cite_note-tsounis-8}
- [↑ 9.0 9.1 Jones, Benji. “Scientists Will Unleash an Army of Crabs to Help Save Florida’s Dying Reef.” Vox, Vox, 27 Sept. 2023, www.vox.com/down-to-earth/2023/9/27/23883039/florida-coral-reef-caribbean-king-crabs-restoration.]{#cite_note-jones-9}
- [↑ 10.0 10.1 10.2 Spadaro, Angelo Jason, and Mark J. Butler. "Herbivorous crabs reverse the seaweed dilemma on coral reefs." Current Biology, vol. 31, no. 4, 10 Dec. 2020, https://doi.org/10.1016/j.cub.2020.10.097.]{#cite_note-spadaro-10}
- [↑ 11.0 11.1 11.2 11.3 Smith, Hillary A., et al. "Sea‐weeding: Manual removal of macroalgae facilitates rapid coral recovery." Journal of Applied Ecology, vol. 60, no. 11, 13 Sept. 2023, pp. 2459–2471, https://doi.org/10.1111/1365-2664.14502.]{#cite_note-smith-11}
- [↑ 12.0 12.1 12.2 12.3 12.4 12.5 Dahlgren, Craig. “Resources.” NSIP, Wildlife Conservation Society, 26 Nov. 2021, programs.wcs.org/Resources/Publications/Publications-Search-II/ctl/view/mid/13340/pubid/DMX4087500000.aspx.]{#cite_note-dahlgren-12}